ELN4EDU-details
Bring an industry-essential asset to your research group
ELN4edu is a platform for recording experiments, building a searchable database, and collaborating on research projects, all supported by our proprietary cheminformatics tools: JSDraw Sketcher and Chrawler Search Engine.
With ELN4edu, bring the benefits of an industry-essential asset to your research group.

Benefits
- Centralized, searchable database
- Built-in cheminformatics
- Upload any data type
- Establish templates and SOP’s to standardize workflows
- Build archives to benefit future researchers
- Improve efficiency in the lab
- Quickly generate presentations reports, and publications
- Collaborate, view data in real-time, and generate feedback

Capture the entire research process in one place
ELN4edu is an ELN tailor-made for academic researchers, ideally suited for smaller, chemistry-focused groups.
Tired of digging through paper notebooks to find that one reaction?
Can’t keep track of all those analytical spectra?
ELN4edu provides an affordable solution to the data silo problem.
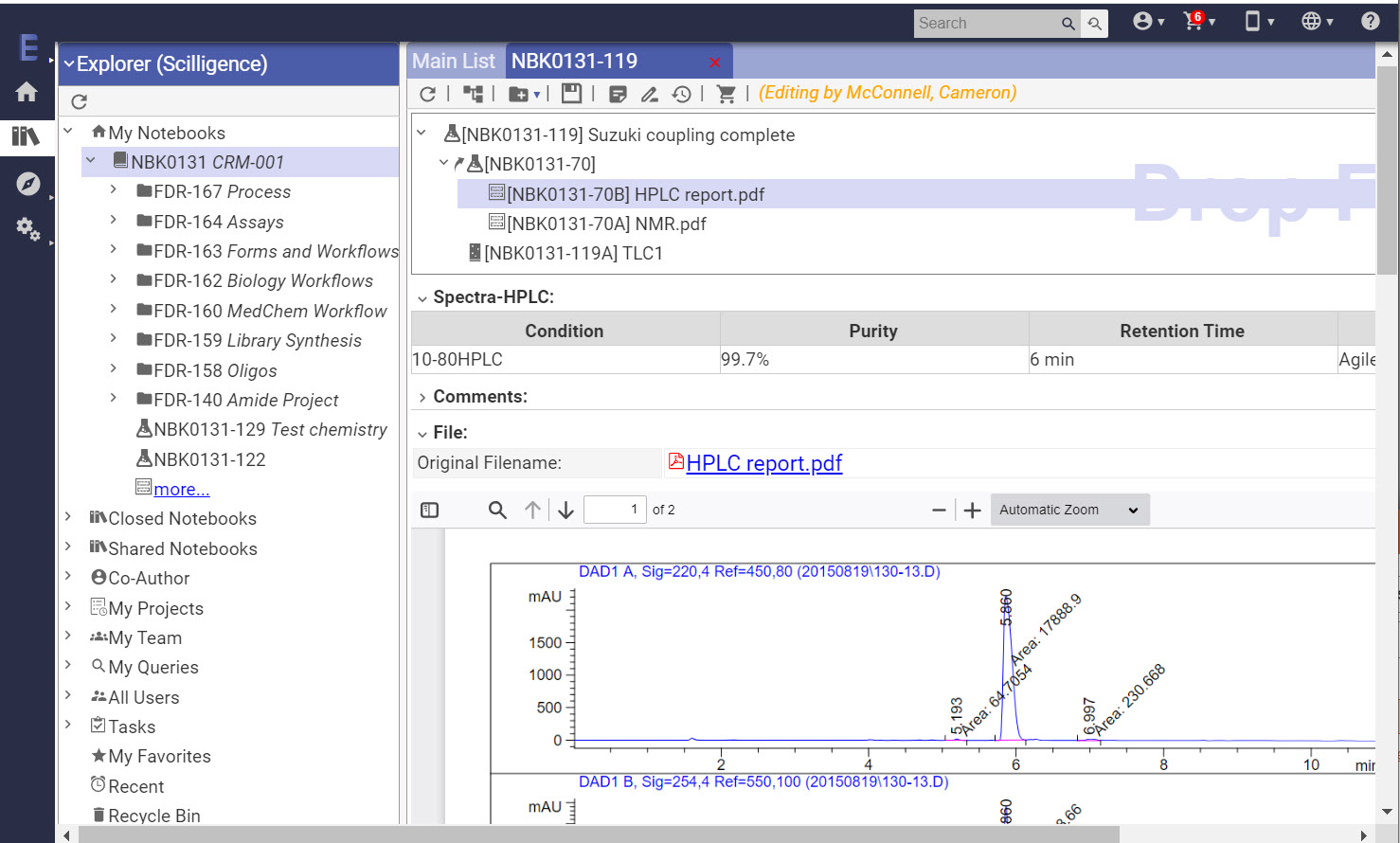
Key Features
- Chemistry reaction templates with built in structure and reaction sketcher
- Stoichiometry tables
- Create, upload, share templates and SOP’s
- Create projects, project documents, manage users and projects
- Reaction and structure search
- View Office, PDF, and image files in-line
- Copy experiments
- Attach any data or file type
- Export or print experiments for reports and publications
- Sign, witness, and close experiments

Would you like a free trial?
Click the link below and fill out the registration form to set up your free trial account.